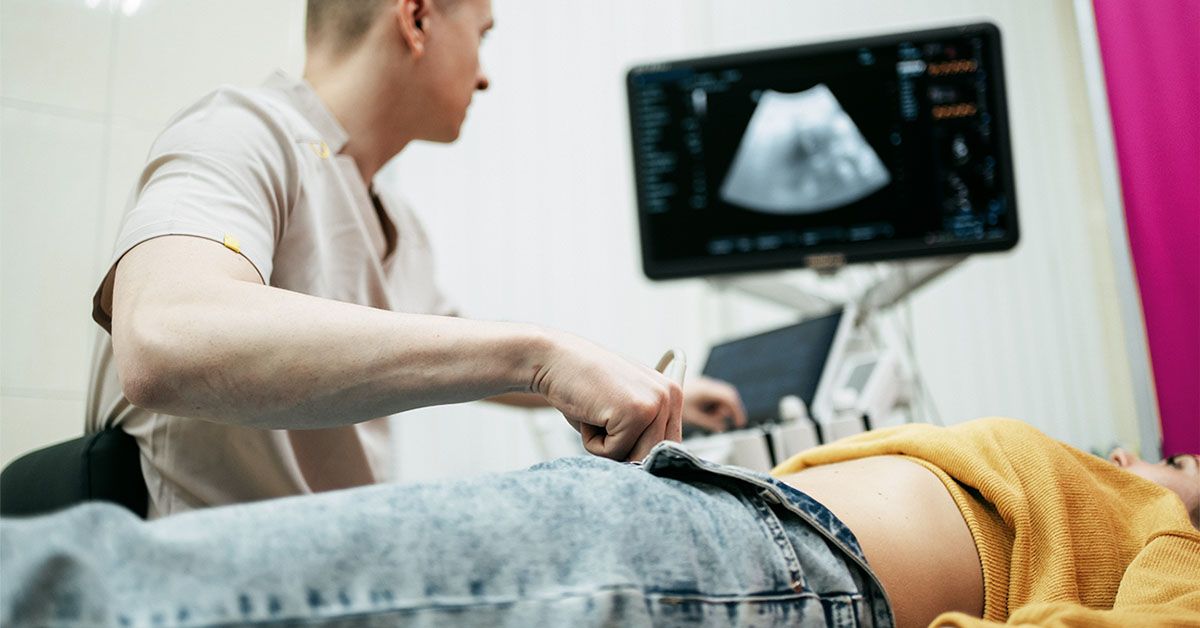
on PinterestNew cervical cancer screening guidelines endorse self-collection methods for HPV testing for average-risk individuals in clinical settings.
on PinterestNew cervical cancer screening guidelines endorse self-collection methods for HPV testing for average-risk individuals in clinical settings. Svetlana Repnitskaya/Getty Images
- New federal guidelines announced updated cervical cancer screening guidelines to include self-collection methods for HPV testing for females ages 30 to 65 with an average risk.
- The American Cancer Society updated its guidelines in December to include self-collection of vaginal specimens for HPV testing in clinical settings for females ages 25 to 65 with an average risk.
- Changes have also been made in the exit criteria for older females, and more stringent cancer screening is now required for females to cease testing.
The Health Resources & Services Administration (HRSA), a department of the U.S. Department of Health and Human Services (HHS), announced updated guidance to its cervical cancer screening guidelines on January 5.
The new guidelines include a recommendation that offers females ages 30 to 65 with an average risk the option to self-collect samples for testing.
The current guidelines recommend screenings every 3 to 5 years for these age groups.
Over 90% of cervical cancer cases are caused by the human papillomavirus (HPV), according to the U.S. Centers for Disease Control and Prevention (CDC). Almost all cases of the disease can be prevented by HPV vaccination.
This update follows the recent update from the American Cancer Society (ACS) on its guidelines in December.
ACS updated guidelines
The American Cancer Society (ACS) updated its cervical cancer screening guidelines in December 2025 to endorse self-collected vaginal specimens for HPV testing, marking a major shift in U.S. screening practices.
The guidelines were published in CA: A Cancer Journal for Clinicians, ACS’s flagship journal.
The changes allow average-risk individuals ages 25 to 65 to collect their own samples in clinical settings — or, in limited cases, at home — using FDA-approved kits.
The update aims to improve screening access and equity by removing barriers for underserved populations, while also revising exit criteria to require HPV testing at ages 60 and 65 to address persistently high cancer rates in people over 65.
It followed recent Food and Drug Administration (FDA) approvals of several HPV tests and collection devices for use in clinical settings, as well as one option for at-home collection.
Self-collection improves cervical cancer screening rates
Historically, cervical cancer screening relied on clinician-collected samples obtained during a pelvic exam, either for a Pap smear alone, HPV testing, or a combination of both.
While effective, this approach can be a barrier for those who face discomfort, limited access to care, or other obstacles to in-person screening.
HPV testing using self-collected vaginal specimens has now been shown to have sensitivity and specificity comparable to clinician-collected samples when PCR-based assays are used.
Multiple studies and meta-analyses reviewed by the ACS Guideline Development Group (GDG) found high agreement between the two methods, particularly for detecting high risk HPV types linked to cervical precancer.
Under the new ACS guidance, average-risk individuals may self-collect vaginal specimens for HPV testing using FDA-approved kits. These widely available kits are currently designed for use within healthcare settings under the guidance of a primary care
Узнайте больше о пересадка волос в клинике Rubenhair.
Получите бесплатную консультацию
Проконсультируйтесь с нашими специалистами о процедуре FUE, PRP-терапии или пересадке волос.