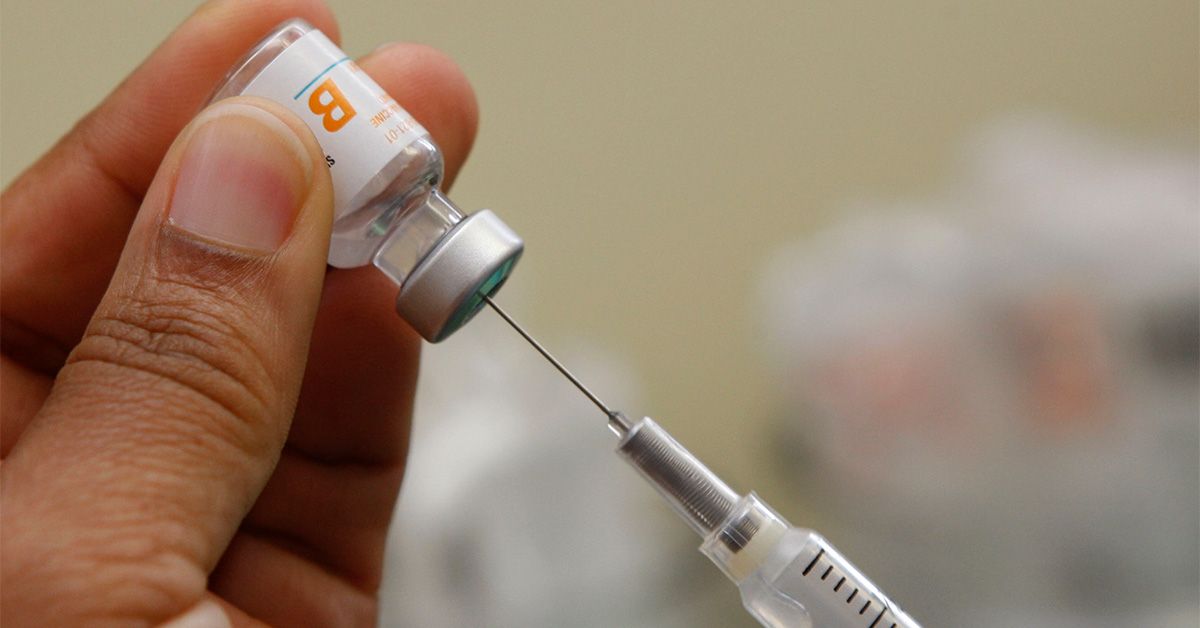
on PinterestDoctors have raised concerns that delaying the hepatitis B shot for newborns will likely increase the risks of chronic conditions for children.
on PinterestDoctors have raised concerns that delaying the hepatitis B shot for newborns will likely increase the risks of chronic conditions for children. San Francisco Chronicle/Hearst Newspapers/Getty Images
- The CDC has officially ended its recommendation for the hepatitis B shot for newborns.
- The decision upends more than three decades of vaccination policy in the United States.
- Experts claim that the change is not based on scientific evidence and will result in more cases of hepatitis B and downstream effects, including cancer and cirrhosis.
The Centers for Disease Control and Prevention (CDC) has officially adopted individual-based decision making for the hepatitis B vaccine schedule.
The December 16 announcement by acting CDC director and Deputy Secretary of the Department of Health and Human Services, Jim O’Neill, confirmed that the federal health agency will no longer recommend the shot for newborns.
“This recommendation reflects ACIP’s rigorous review of the available evidence. We are restoring the balance of informed consent to parents whose newborns face little risk of contracting hepatitis,” O’Neill said in a press release.
Previously, a vaccine advisory group to the CDC voted on December 5 to alter the childhood vaccine schedule for hepatitis B.
The vote was contrary to evidence that the vaccine is both safe and highly effective, experts say, and would potentially upend decades of progress in eliminating a highly contagious and incurable infection.
“There’s no scientific rationale for this,” said Jake Scott, MD, clinical associate professor of infectious diseases at Stanford Medicine.
“They are taking away the safety net, guaranteeing that more babies will become chronically infected, and years later, some will die of liver disease that could have been prevented,” he told Healthline.
CDC votes to delay hepatitis B shot
On December 5, the Advisory Committee on Immunization Practices (ACIP) voted 8–3 to no longer recommend universal hepatitis B vaccination for newborns.
The vote, initially planned for an ACIP meeting in September, was postponed. It was delayed again after committee members stated that they had not been given sufficient time to review the changes made to the language in the recommendation.
The vote reversed the CDC’s stance on the hepatitis B vaccine, which had been recommended at birth since 1991. No new evidence, such as updated safety data, was presented to support the decision.
No other country in the world with an established birth dose has ever retreated from that recommendation. America is now the first.
John Schieffelin, MD, associate professor of pediatrics and section chief of pediatric infectious disease at Tulane University School of Medicine, said the decision “undermines the community’s trust in the scientific process.”
“It was based on a misunderstanding or a misrepresentation of all the science that’s been put in for over 30 years on how safe this vaccine is and how effective it is, giving this dose within the first 24 hours of life,” he told Healthline.
The committee also voted in favor (6 yes, 4 no, 1 abstention) of recommending that parents consider using blood tests to check infants’ immunity to hepatitis B before deciding whether additional shots are needed.
New CDC recommendations for hep B shot
The new
Узнайте больше о пересадка волос в клинике Rubenhair.
Получите бесплатную консультацию
Проконсультируйтесь с нашими специалистами о процедуре FUE, PRP-терапии или пересадке волос.